A recent medical study shows a five-fold increase in brain meningiomas in Depo-Provera users. Also known by the chemical name medroxyprogesterone acetate, Depo-Provera is a widely used birth control in the United States and Europe. Women receive intramuscular injections every three months. Many women use it for an extended period of time and are at particular risk for tumors. The FDA originally denied it for birth control use due to the lack of safety studies, but Depo-Provera finally got approved in 1992.
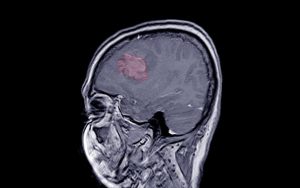
Litigation will focus on why no warnings were added even though the meningioma link has been known for decades. Since the 1980s meningiomas have been suspected to be more common in women using synthetic progestogen medicines. Progestogen increases the growth potential of tumors because meningioma cells have progesterone receptors. Medical articles published in 2022 and 2023 have linked Depo-Provera to an increased incidence of meningiomas.
If you have developed a meningioma after taking Depo-Provera, we urge you to contact our firm for a free consultation.
Our firm offers lower fees on settlement values than most competitors. We work on a contingency basis.
While the United States products do not have meningioma warnings, other countries have a “Special Warnings and Precaution” section stating, “meningiomas have been reported following long-term administration of progestogens.” Progestogens are a synthetic and stronger form of natural progesterone.
Also, there is a safer, lower dose version of Depo-Provera which is injected with a smaller needle into the skin surface and not deep into muscles. The product is known as “Sayana Press” in Europe and patients can inject themselves. Litigation will focus on why the companies did not promote this safer product.
Meningiomas are generally slow-growing, non-cancerous tumors that grow in the meninges (fluid-filled spaces in the brain). Symptoms range from asymptomatic to life-threatening complications. Removed tumors can be submitted to pathology labs where they are stained to reveal the presence of progestogens—the active ingredient in Depo-Provera. Our firm is determining what types of tumors are likely related to Depo-Provera.
Litigation will involve women who have used the medicine for at least a year and who underwent meningioma surgery, or who have a meningioma and are experiencing complications. Some tumors are inoperable if they are at the base of the skull, near eye arteries, or deep in the brain.
Settlement compensation will take into account everything that a woman has been through, including doctors’ visits, surgeries, procedures, pain and suffering, and lost wages.
Before surgical intervention, injuries can include:
Any brain tumor is a serious injury. Surgeons can use an MRI, CT scan, X-ray, MRA or PET scans to study the size and location of the tumor. They are also useful methods after surgery to monitor progress. Some tumors are discovered that present an emergency situation because they are causing permanent harm. Unfortunately, some tumors cannot be removed by surgery.
The shockingly high rate of meningiomas with the total lack of warnings to patients will lead to a flood of lawsuits. Certain states and federal courts are expected to become places for consolidated litigations. Where suits get filed will depend on where the injured woman is from and what drug manufacturer made the Depo-Provera. The medicine was produced by spin-off companies and authorized generics manufacturers. Law firms will track down which company made the Depo-Provera that was injected. Depending on the year and location where the Depo-Provera was used, it may have been sold by one or more of these companies:
Our firm has litigated against many hormonal birth control products, including Ortho Evra patch, Norplant, and NuvaRing. We have also litigated IUD birth control products, such as Dalkon Shield, Mirena and ParaGard. These litigation experiences prepare our firm to take on this litigation and advocate for the victims of injuries.
Our team serves people injured by defective drugs across the nation. Call (212) 684-1880 to learn more during a free and confidential consultation.
We fight tirelessly to deliver results and we are not afraid to go to trial.
We are leaders and educators in legal issues involving personal injury, medical malpractice, and product liability.
We have the experience to handle your case all while providing individualized attention.
Trustindex verifies that the original source of the review is Google. Tom Giuffra was effective and professional on my recent case; he got a better outcome than I was hoping. Very positive experience and highly recommend.Trustindex verifies that the original source of the review is Google. I would recommend Edward Ruffo to the highest degree possible for anyone in need of a top-notch medical malpractice lawyer. Over the past six years, he has provided me with exceptional service and support. From day one, Mr Ruffo was incredibly attentive, always taking the time to explain complex legal processes in a way that I could understand. Moreover, he even gave me his personal cell phone. Allowing me to reach out, whenever I had questions or needed further clarity. What truly sets Mr Ruffo apart though, is how tirelessly he fought on my behalf. He went above and beyond in all matters, and allowed me to focus on my health while he took care of the rest. If you're looking for a lawyer who will truly go the extra mile for you, look no further.Trustindex verifies that the original source of the review is Google. Edward Ruffo was the lawyer I chose to represent my family after losing my mother to nursing home negligence resulting in her death.From the first time I spoke to him, he demonstrated a true passion for his job. He also demonstrated a strong ability and willingness to listen, displayed an excellent knowledge of the law, possesed excellent communication skills, was very patient, hardworking and persevering. His expertise in the area of law that he practices is superb. He is informative and is available whenever I need to speak to him or his staff. Any information imparted to me is easy to comprehend and explained in understandable terms. Mr Ruffo has worked tirelessly on my mother's case and everyone at his firm has been kind, patient and supportive. Mr Ruffo's paralegal , Nathalia Eusebio is one of the most kindhearted and personable people that I have ever had the priviledge of talking to. I cannot wait to meet her in person. She is always available to answer any questions that I have when I call the office, is friendly, knowledgeable and professional. I would recommend this attorney group to anyone who requires assistance with litigation.Trustindex verifies that the original source of the review is Google. Tom Giuffra is truly one of the very best! He gets huge verdicts for his clients and I would highly recommend him.Trustindex verifies that the original source of the review is Google. Mr.Giuffra helped me quickly reach a happy ending to a difficult time. He handled everything with the utmost professionalism and quickly/efficiently a resolution was established.Trustindex verifies that the original source of the review is Google. I completely recommend working with this firm. I specifically worked with Tom and I’m very appreciative to him for handling my case and getting me a positive outcome.Trustindex verifies that the original source of the review is Google. Thank u to Tom and Victoria for all they have done for me. They did an amazing job!!!!