Our firm is now litigation hundreds of suits against Johnson & Johnson for defective metal-on-metal implants. Their subsidiary, DePuy, marketed these implants to doctors as
In January we started a federal court suit on behalf of a Virginia woman who was injured through the use of the DePuy Pinnacle Ultamet
Personal-care-product giant Johnson & Johnson (J&J) was recently on the receiving end of a scathing letter from the U.S. Food and Drug Administration about faulty
Back in 2006, Wright Medal Inc. took steps to convince the FDA to reject pre-market approval of rival Smith & Nephew’s Birmingham metal-on-metal hip implant.
For patients who allow their doctors to implant medical devices in their bodies, it is a reasonable expectation that those devices have been thoroughly tested
Many women have a Paragard implanted which is at or near its life expectancy. The device was originally approved for six years of use but
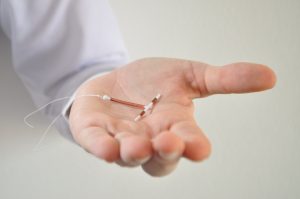
La FDA ha enviado una carta al fabricante del dispositivo anticonceptivo Paragard advirtiéndole que no puede omitir los riesgos que supone el uso del dispositivo.
The FDA has sent a warning letter to the manufacturer of the contraceptive device Paragard concerning its omission of risks with the device. CooperSurgical failed
In what could be called “too little, too late” for the impotent and corporately-influenced FDA, the orthopedic division has asked to impanel a medical-science committee
A new study recently published by the FDA ahead of a meeting of the Reproductive Health Drugs Advisory Committee and the Drug Safety and Risk
In a recent update U.S. District Judge Leigh May Martin announces monthly status conferences schedule for Paragard injury lawyers with multi-district litigation (MDL) court. The
With more than 100 Paragard lawsuits pending in the federal multidistrict litigation (MDL), the presiding judge, Leigh Martin May, has ruled that plaintiffs should file
When DePuy Orthopedics issued recalls for the ASR XL Acetabular Hip Replacement System and the ASR Hip Resurfacing System last August, the company said data
The U.S. Food & Drug Administration (FDA) has set up a website for metal-on-metal hip implants, which among other things, details the potential risks posed
Recently, a panel of federal judges decided to consolidate all DePuy Pinnacle hip implant lawsuits to a multi-district litigation and transferred it to the federal
Recently, five United States senators reached out to the Inspector General of the Department of Health and Human Services, seeking an investigation into physician-owned distributorships,
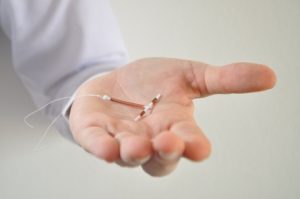
Lawsuits have started for women who have suffered broken IUD arms during removal of their Paragard devices. The litigation just got a big “green light”
A recent New York Times article highlighted the growing problems with Metal on Metal implants, such as the DePuy ASR and Zimmer Durom Cup, and what is
In July 2011 the Institute of Medicine (IOM), the medical branch of the National Academy of Sciences, joined the group of voices calling on the
Before pacemakers, stents and other medical devices for the heart are approved for use by the U.S. Food and Drug Administration, the devises must be put through
The Government Accountability Office (GAO) recently released its evaluation on how the FDA has responded to the GAO’s 2009 findings on the shortcomings of the FDA’s premarket
A warning letter issued by the FDA on August 19, 2010 to DePuy Orthopaedics cites numerous cases where DePuy marketed two hip replacement systems without
Less than half of surgeons who recieved $1 million or more in payments from medical device manufacturers disclosed their relationships when publishing scientific articles according to a
WRITTEN BY PAUL D. RHEINGOLD The NuvaRing birth control litigation has taken a major step forward with the selection of cases which will be tried first. These
After the long wait since the October, 2009, proposal for consolidated litigation, the decision has been made to consolidate the cases before Judge Brian R.
The Judicial Panel on Multidistrict Litigation has chosen to consolidate the DePuy ASR litigation into the the Northern District of Ohio under Judge Katz. This will allow
External defibrillators are medical devices for use on people who collapse because of sudden cardiac arrest. In an emergency, the external defibrillator should restore heart rhythm with
The FDA sent out an advisory to doctors and patients about a medical device that is used to prevent blood clots from traveling to the lungs. These
Surgical staplers are devices used to cut and staple internal tissue throughout a wide variety of surgeries. Unfortunately, between January 1st, 2011 and March 31st,
On October 7th, 2021 the U.S. Food and Drug Administration (FDA) released a statement providing additional recommendations for health care providers in order to protect
On December 21st, the FDA approved the Philips CavaClear Laser Sheath for use with conventional snare devices for the removal of inferior vena cava (IFC)
Medtronic, the Irish medical device manufacturer whose headquarters in the US are in Minneapolis, is facing renewed scrutiny concerning two of its products-the Medtronic Harmony