If you or a loved one have been injured by a CR Bard hernia mesh device, the time to act is now. With 12,000 lawsuits against
Recently, a panel of federal judges decided to consolidate all DePuy Pinnacle hip implant lawsuits to a multi-district litigation and transferred it to the federal
Recently, five United States senators reached out to the Inspector General of the Department of Health and Human Services, seeking an investigation into physician-owned distributorships,
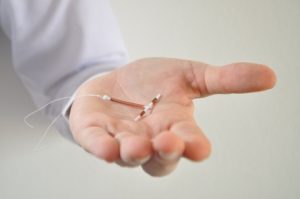
Lawsuits have started for women who have suffered broken IUD arms during removal of their Paragard devices. The litigation just got a big “green light”
On February 4, 2011, Judge Mary M. Lisi, of the U.S. District Court for the District of Rhode Island, denied C.R. Bard Inc.’s (Bard) motion
When Linda Gross’ doctor advised her to have surgery to correct her pelvic organ prolapse (POP), she could not have suspected the complications that would
A non-profit advocacy group, Public Citizen, is strongly encouraging the FDA to remove from the market synthetic surgical mesh products that treat a condition called
A recent New York Times article highlighted the growing problems with Metal on Metal implants, such as the DePuy ASR and Zimmer Durom Cup, and what is
In July 2011 the Institute of Medicine (IOM), the medical branch of the National Academy of Sciences, joined the group of voices calling on the
Before pacemakers, stents and other medical devices for the heart are approved for use by the U.S. Food and Drug Administration, the devises must be put through
The Government Accountability Office (GAO) recently released its evaluation on how the FDA has responded to the GAO’s 2009 findings on the shortcomings of the FDA’s premarket
A warning letter issued by the FDA on August 19, 2010 to DePuy Orthopaedics cites numerous cases where DePuy marketed two hip replacement systems without
U.S. District Judge Edmund A. Sargus, Jr. in the Southern District of Ohio has denied Bard’s summary judgement motion which clears the way for all
Less than half of surgeons who recieved $1 million or more in payments from medical device manufacturers disclosed their relationships when publishing scientific articles according to a
In July 2007, John Doe entered the hospital for a routine umbilical hernia surgery. Other than the hernia issue, he was a healthy male who
WRITTEN BY PAUL D. RHEINGOLD The NuvaRing birth control litigation has taken a major step forward with the selection of cases which will be tried first. These
With more than 8,000 lawsuits already filed against C.R. Bard over defective hernia mesh devices, the company does not need another headache from their product
In the middle of the Bard hernia mesh bellwether trial, lawyers for the medical device manufacturer introduced a motion that would have excluded crucial evidence
After the long wait since the October, 2009, proposal for consolidated litigation, the decision has been made to consolidate the cases before Judge Brian R.
The Judicial Panel on Multidistrict Litigation has chosen to consolidate the DePuy ASR litigation into the the Northern District of Ohio under Judge Katz. This will allow
External defibrillators are medical devices for use on people who collapse because of sudden cardiac arrest. In an emergency, the external defibrillator should restore heart rhythm with
The FDA sent out an advisory to doctors and patients about a medical device that is used to prevent blood clots from traveling to the lungs. These
A federal jury has ordered that CR Bard must pay $255,000 to a hernia mesh recipient. It represents a significant victory in the Bard hernia
A Rhode Island jury has heard both sides in the lawsuits involving CR Bard’s Ventralex hernia mesh. Plaintiffs argued that the Bard Ventralex’s design of
For Paul Trevino and his family the suffering caused by the defective hernia mesh has been vindicated by a Rhode Island jury’s verdict of $4.8
Surgical staplers are devices used to cut and staple internal tissue throughout a wide variety of surgeries. Unfortunately, between January 1st, 2011 and March 31st,
The second bellwether trial in the Multidistrict Litigation (MDL) concerning the Bard Ventralex hernia mesh is set to begin on March 21, 2022. The judge
On October 7th, 2021 the U.S. Food and Drug Administration (FDA) released a statement providing additional recommendations for health care providers in order to protect
What is a hernia? A hernia is a medical condition that takes place when an internal organ protrudes via the abdominal wall of the tissue
The Hernia Mesh Litigation filed by Antonio Milanesi and Alicia Morz De Milanesi is scheduled to begin this Monday on January 10th. The trial is
On December 21st, the FDA approved the Philips CavaClear Laser Sheath for use with conventional snare devices for the removal of inferior vena cava (IFC)
Medical device manufacturer Exactech has been operating since 1985 with global sales of their hip, ankle, and knee replacement devices. A massive recall has been
Medtronic, the Irish medical device manufacturer whose headquarters in the US are in Minneapolis, is facing renewed scrutiny concerning two of its products-the Medtronic Harmony